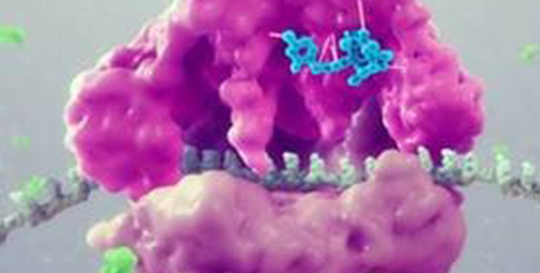
This semi-synthetic compound inhibits the synthesis of bacterial protein, which is required for bacteria to grow. It acts by binding to the peptidyl transferase center, or PTC, on the bacterial ribosome in such a way that it interferes with the interaction of protein production at two key sites known as the “A” site and the “P” site, resulting in the inhibition of bacterial proteins and the cessation of bacterial growth. Its binding occurs with high affinity, high specificity and at molecular sites that are different than other antibiotic classes.
We have completed two pivotal Phase 3 trials evaluating the safety and efficacy of lefamulin in for the treatment of adults with community-acquired bacterial pneumonia (CABP). In our first clinical trial in patients with CABP (LEAP-1), seven-days of intravenous (IV) to oral lefamulin in adults with moderate to severe CABP was compared to moxifloxacin (IV/oral), with or without linezolid. In the LEAP 2 trial, five-days of oral lefamulin was compared to seven-days of oral moxifloxacin in adults with moderate CABP. Both trials were multi-center, randomized, controlled, double-blind and enrolled patients globally. Lefamulin met all U.S. Food and Drug Administration (FDA) and European Medicines Agency (EMA) primary endpoints in both LEAP 1 and LEAP 2 and was shown to be generally well tolerated. As a result of the favorable safety and tolerability profile we have observed in our clinical trials to date, we believe Lefamulin will present fewer potential complications as than current therapies. In December 2018, we completed the submission of two New Drug Applications (NDAs) to the FDA for the oral and IV formulations of lefamulin for the treatment of CABP. In February 2019, these applications were accepted by the FDA, granted priority review and given a PDUFA date of August 19, 2019. Both formulations of lefamulin were granted Qualified Infectious Disease Product (QIDP) and Fast Track designation by the FDA. On August 19, 2019, the FDA Approved Xenleta™(lefamulin) for both Oral And IV use. Nabriva intends to work with a commercial partner to make lefamulin available to patients in the European Union. In July 2020, the European Commission issued a legally binding decision for approval of the marketing authorization application for XENLETA™ (lefamulin) for the treatment of community-acquired pneumonia in adults following a review by the European Medicines Agency. In July 2020, our partner, Sunovion Pharmaceuticals Canada Inc., received approval for Xenleta®(lefamulin) from Health Canada for the treatment of community-acquired pneumonia (CAP) in adults.
Based on our research, we also believe that the availability of both IV and oral formulations of lefamulin, and an option to switch to oral treatment, could reduce the length of a patient’s hospital stay and the overall cost of care. Based on a combined analysis of the U.S. Centers for Disease Control and Prevention’s 2007 National Ambulatory Medical Care Survey and 2013 data from the Healthcare Cost and Utilization Project, Nabriva Therapeutics estimates that more than 5 million adults visit a site of care for CABP treatment in the United States each year. Based on 2013 data from the Healthcare Cost and Utilization Project, Nabriva Therapeutics estimates that approximately 3 million of these adult CABP patients are diagnosed in a hospital setting, where most are then treated as in-patients with IV and oral antibiotics or as Transition of Care out-patients with oral antibiotics following hospital discharge or release. To learn more about CABP and why new treatments are needed, click here.
Nabriva owns exclusive, worldwide rights to lefamulin.
We intend to further pursue the development of this antibiotic and are developing a formulation that is also appropriate for pediatric use. We believe that lefamulin's product profile also provides the opportunity to expand to additional indications beyond pneumonia, such as the treatment of acute bacterial skin and skin structure infections (ABSSSI), sexually transmitted infections (STIs), ventilator-associated bacterial pneumonia (VABP), hospital-acquired bacterial pneumonia (HABP), osteomyelitis, and prosthetic joint infections.